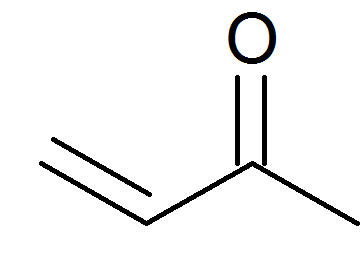
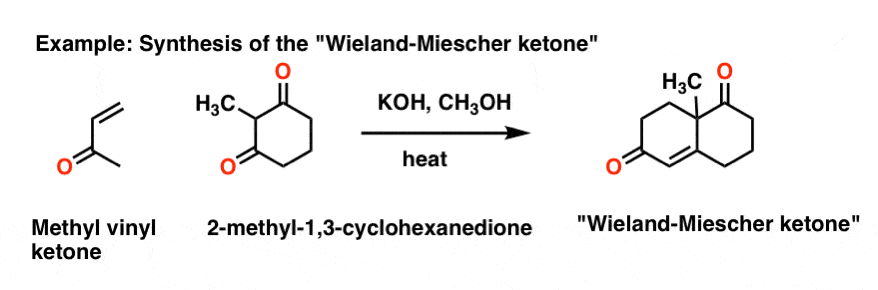
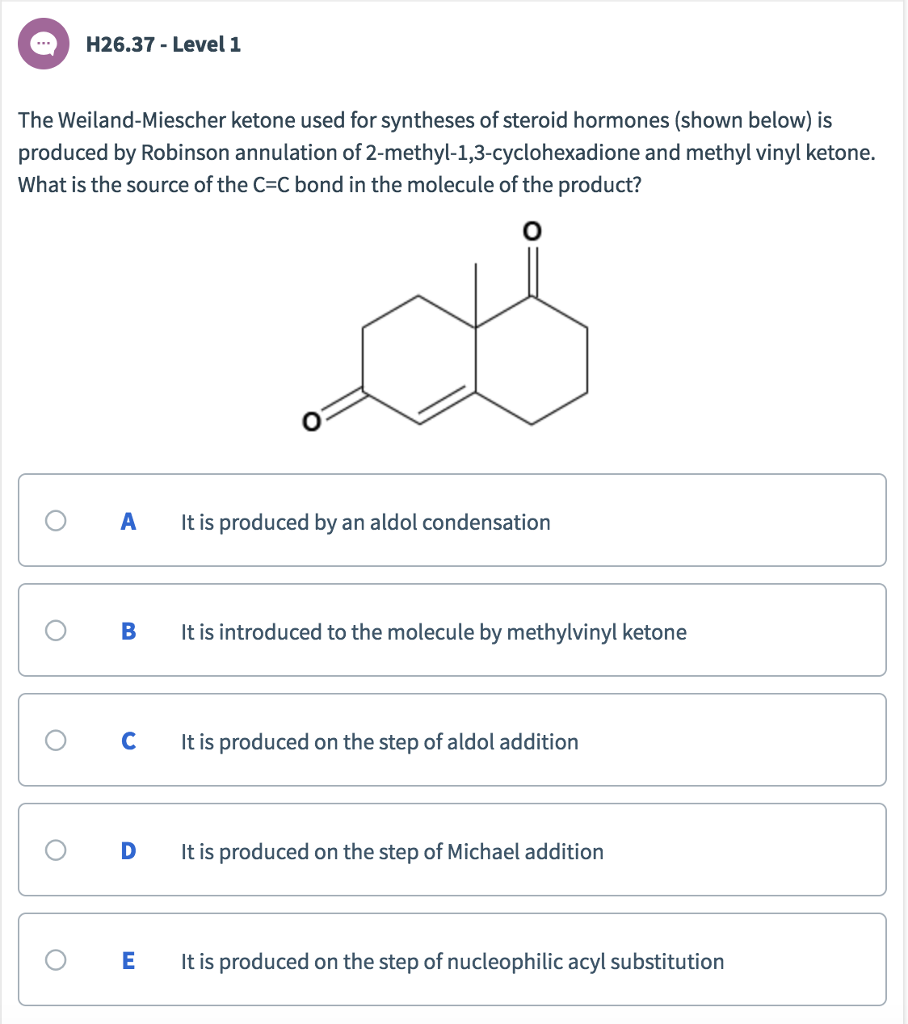
Michael Addition Methyl Vinyl Ketone And

Nitropropane with methyl vinyl ketone.
Michael addition methyl vinyl ketone and. Diethyl malonate with methyl crotonate. In this approach the di tri and tetrasubstituted propargylamines are synthesized in 46 98 yields. Diethyl malonate with diethyl fumarate. Michael addition to methyl acrylate and methyl vinyl ketone of n b benzylidene l tryptophan methyl ester 1 gave 2 3 indolylmethyl glutamic dimethyl ester 2a and α 3 oxobutyl tryptophan methyl ester 2b respectively.
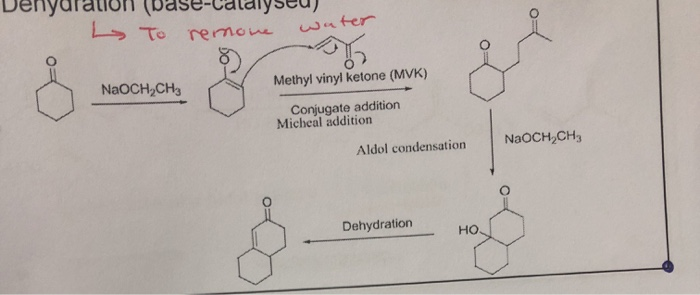
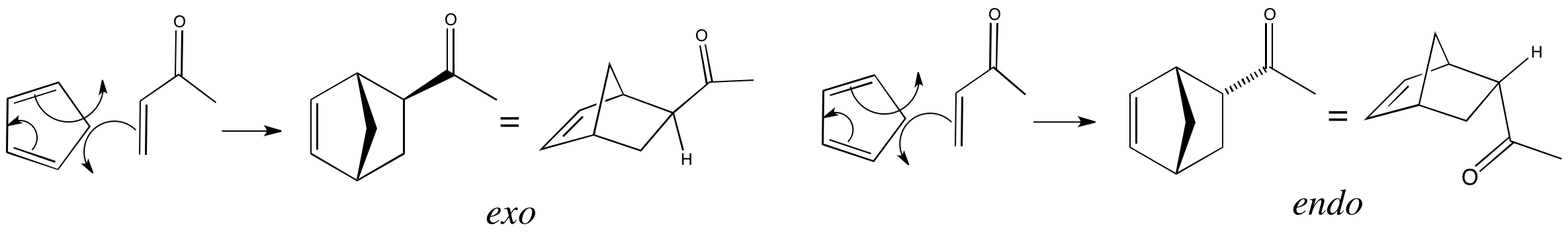
The robinson annulation is a chemical reaction used in organic chemistry for ring formation. The michael reaction or michael addition is the nucleophilic addition of a carbanion or another nucleophile to an α β unsaturated carbonyl compound containing an electron withdrawing group it belongs to the larger class of conjugate additions this is one of the most useful methods for the mild formation of c c bonds. Michael addition mechanism step 1. In this scheme r and r on the.
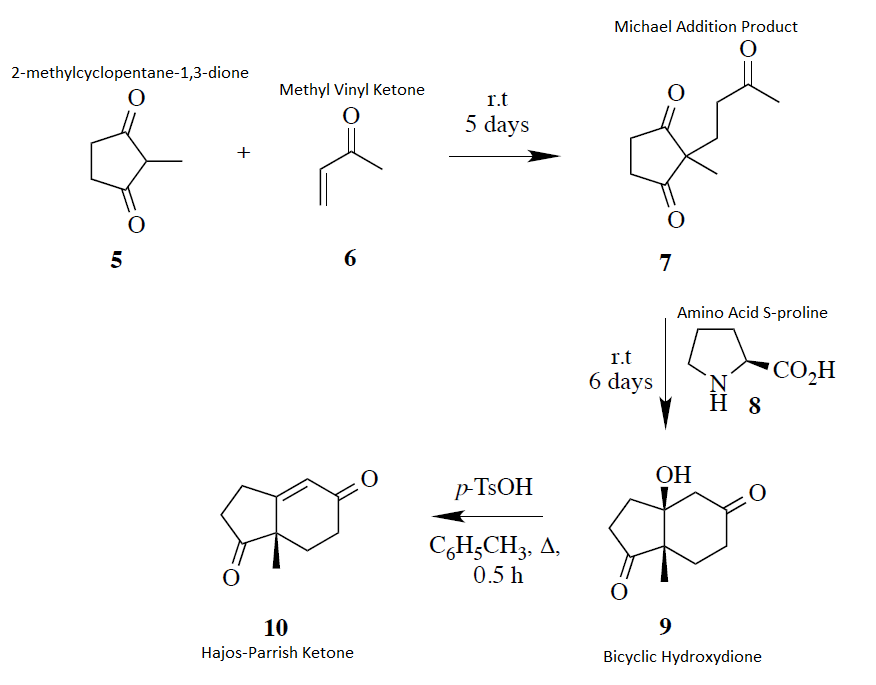
Hydrolysis of ester groups and decarboxylation occurs in the final step. Mesityl oxide with diethyl malonate. The carbonyl containing compound is attacked by the base in the first step of the michael addition mechanism. Propargylamines are synthesized from methyl vinyl ketone derivatives 1 alkynes and secondary amines catalyzed by cu salts involving the michael addition of amine followed by an unusual c c bond cleavage and addition of metal acetylides formed in situ to iminium ions.
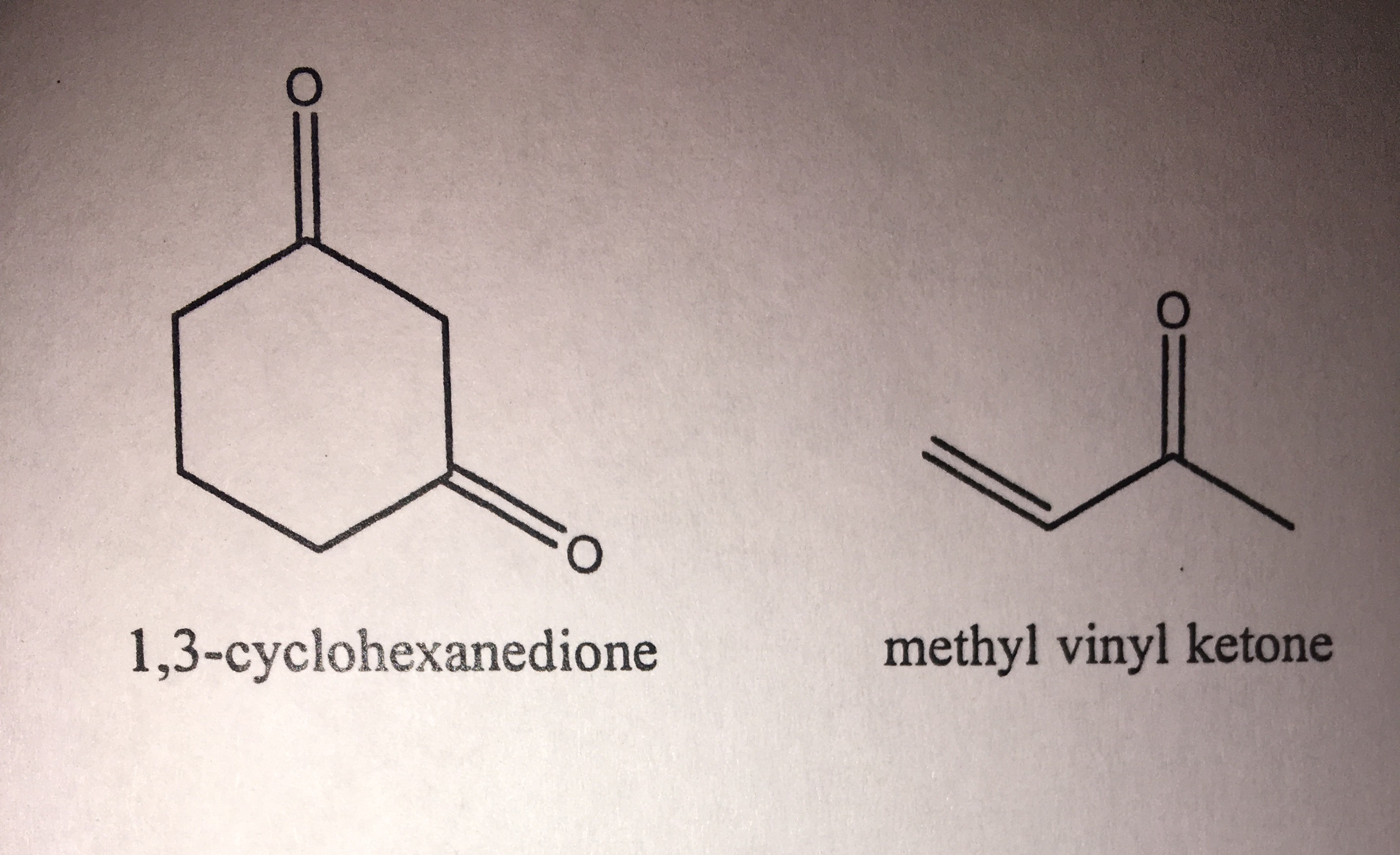
Many asymmetric variants exist. But a kinetically controlled 1 2 addition is also possible which is preferred at low temperatures as shown below. 1 michael addition of diethyl malonate with methyl vinyl ketone followed by protic workup yields a 1 5 dicarbonyl compound. 2 nitropropane with methyl acrylate.